Article reproduced from Wing Beats, the bulletin of the American Mosquito Control Association, produced by the Florida Mosquito Control Association. Please use the following citation when referring to this article:
O'Malley, C. 1995. Seven ways to a successful dipping career. Wing Beats, vol. 6(4): 23-24.
CLAUDIA O'MALLEY
Introduction
As recently as 1922, members of the New Jersey mosquito control community were debating the relative merits of "night collections" as opposed to larval collection and identification. Some of the past practices of mosquito control included treating any standing water encountered, regardless of whether or not mosquito larvae were present. Guidelines for starting a mosquito control program included the advice that directors should not spend an excessive amount of time on surveys. Even now, a few still feel that larval surveys are only necessary in the early part of the breeding season; once it is known what species are present at a site, it can be taken for granted that the species composition at that site will remain the same throughout the rest of the season. Most experts, however, feel that larval surveillance is not only an important aspect of an effective mosquito surveillance and control program, but it is an essential component.
Benefits of Larval Surveillance
Larval surveys have many important functions. They are used to determine the locations and seasons that mosquitoes use specific aquatic habitats and, when specimens are identified and counted, the information can be used to determine species composition and population densities. The information can be used to determine optimal times for application of larval control measures, including chemicals, biologicals, draining or impounding. It can also be used to help forecast the need for adult mosquito control and to help assess the effectiveness of both chemical and biological control measures.
Routine larval surveillance data can be useful in interpreting adult mosquito surveillance data. For example, if larval surveys indicate 95- 1 00% control by larvicides and yet the number of adults does not decline, one can suspect, in the absence of reinfestation, that an important larval concentration was missed. A system for the detection of insecticide resistance is also provided through a larval surveillance program.
Sampling Larval Mosquitoes
Because mosquito larvae are found in a wide variety of habitats, a number of different sampling techniques to determine their presence and density have been developed. Many, if not all, of the published methods are described in Mike Service's book, Mosquito Ecology Field Sampling Methods (Elsevier Applied Science, 1993).. Some methods are complex mechanical devices, but the most commonly used larval collection method is the "standard dipper," that plastic or metal, white or aluminum, solid or screen-bottomed pint to quart-sized scoop-on-a-handle, that, along with the "sweep net," defines the Ultimate Inspector. Let's take a closer look at dipping.
Dipping for mosquito larvae may, at first, seem like a very simple thing to do. After all, who hasn't dipped water from a bucket or stream to quench a thirst or cool the top of one's head? Well, think again. Dipping for mosquito larvae is not dipping to take a drink. The technique starts long before the dipper is put into the water. It begins hours or days before the actual dipping and at least 10 feet away from the water's edge.
The species of mosquitoes one is looking for and the type of habitat being sampled will, in part, determine the sampling method used. Thus, it is important that field personnel know the preferred breeding habitats and seasonal occurrence of species known or suspected to be present within an area.
When searching for mosquito larvae, proceed slowly and carefully. Approach the area with caution, not to avoid snakes, although that's a good idea too, but to avoid disturbing larvae at the water's surface. Vibrations from heavy footsteps, casting a shadow or moving vegetation that contacts the water may be enough to cause larvae to dive to the bottom. Try to approach the water while facing the sun and with quiet, slow, soft steps, gently move vegetation only as necessary.
Mosquito larvae of most genera, particularly the common Culex, Aedes and Anopheles, are usually found at the water's surface and frequently next to vegetation or surface debris. In larger pools and ponds, they are usually near the margins, not in open, deep water. Dipping should be concentrated around floating debris and aquatic and emergent vegetation. If there is a strong wind, dipping should be done on the windward side of the habitat where larvae and pupae will be most heavily concentrated. Look for larvae and pupae before beginning to dip, if possible. If it is raining on the water's surface, get back in the truck, go have a cup of coffee and wait until the rain stops.
Each water body may contain a number of different microhabitats which could contain different mosquito species. Microhabitats are such places as under tree roots, within clumps of emergent vegetation, under floating or overhanging vegetation and in open water. Learn to recognize different microhabitats within an area and sample as many as possible in order to obtain an accurate picture of the area's species composition.
A Choice Of Seven
Now that you've found your way safely to the edge of a marsh, pond, ditch, swamp or woodland pool, what do you do with your dipper. Just plunge it in? That's fine if you need water, but not necessarily if you want to catch mosquitoes. Believe it or not, there are seven basic ways to dip for mosquito larvae. Which one or ones you use depend, as we mentioned earlier, on the genus or genera of mosquitoes you suspect may be present and on the habitat, microhabitat and weather conditions.
The first and usually the best method to start with is the SHALLOW SKIM. The shallow skim consists of submerging the leading edge of the dipper, tipped about 45 degrees, about an inch below the surface of the water and quickly, but gently, moving the dipper along a straight line in open water or in water with small floating debris. End the stroke just before the dipper is filled to prevent overflowing. The shallow skim is particularly effective for Anopheles larvae that tend to remain at the surface longer than Aedes and Culex. Anopheles are usually associated with floating vegetation and debris.
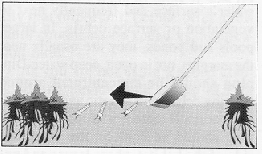
The second method to try in open water, with or without floating objects, is the COMPLETE SUBMERSION. Many mosquito larvae, particularly those of the genera Aedes and Psorophora, are very active and usually dive below the surface quickly if disturbed. In this case, a quick plunge of the dipper below the surface of the water is required, bringing the dipper back up through the diving larvae. Bring the dipper up carefully to avoid losing the larvae in the overflow current.
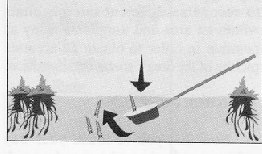
When you need to sample at the edges of emergent vegetation, try the PARTIAL SUBMERSION technique. To do this, push the dipper, tilted at about 45 degrees, straight down adjacent to the vegetation. This causes the water around the vegetation to flow into the dipper, carrying the larvae with the flow. There is no need to move the dipper horizontally. Pull the dipper up before it is full.
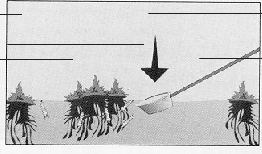
In very shallow water, try the FLOW-IN method. Larvae can be collected by pushing the dipper into the substrate of the pool and letting the shallow surface water, debris and larvae flow into the dipper. Do not move the dipper horizontally.

To sample for larvae that may be under floating or emergent vegetation, use the SCRAPING technique. This method is used in habitats that contain clumps of vegetation such as tussocks of sedges, floating mats of cattails or water lettuce or other plants that are too large to get in the dipper, or clumps of submerged vegetation such as hydrilla or bladderwort. Dip from the water in towards the vegetation and end by using the dipper to scrape up against the base or underside of the vegetation to dislodge larvae. This method is usually more effective if the bottom of the dipper is screened and it is often used to sample for Coquillettidia and Mansonia mosquitoes.
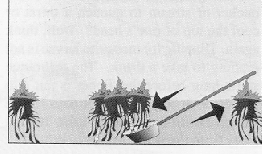
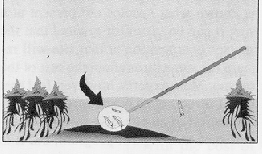
The SIMPLE SCOOP is the "dipping to get water" method that was discouraged earlier. It consists of simply scooping a dipperful of water. This is probably the most commonly used method, particularly by new inspectors, and it is often the method referred to in much of the literature as "the standard dipping procedure." While it can be successfully used to collect Culexlarvae, it is still not the method of choice.
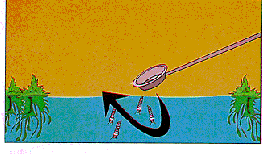
The dipper can also be used as BACKGROUND. This is especially useful in woodland pools and other shallow water or when larvae are disturbed and dive to the bottom. Submerge the dipper completely to the bottom litter and slowly move it around. The darker mosquito larvae and pupae will stand out against the background of a white or aluminum dipper. Once larvae appear in the dipper, just lift it upward.
One or more of these methods, properly used, can determine the mosquito species composition of most aquatic habitats, excluding those whose openings are smaller than the dipper, such as tires, rock pools, treeholes and tree root systems like those found in cedar and red maple swamps. In those cases, a smaller container, such as a vial, measuring spoon or tea strainer can be used in the same seven ways as the dipper described above. Then there is the tubular dipper, the chef's poultry baster, for those really hard to get to places like plant axils, treeholes and tree root holes.
Now that we know how to efficiently collect mosquito larvae, what do we do with the specimens and the data. That's the subject of a future article. Until then, happy dipping.
Claudia O'Malley is with the Burlington County Mosquito Extermination Commission in New Gretna, N.J.
